Atomic Mass of Aluminium Atomic mass of Aluminium is 26.9815 u. The number of protons in the nucleus is called the atomic number. The atomic number of each element is unique. The combined number of protons and neutrons in an atom is called the atomic mass number. While the atomic number always stays the same some elements have atoms with different atomic mass numbers. Notes on the Atomic Mass of particular elements: Technetium: Atomic mass number given for longest lived isotope. Polonium: Atomic mass number given for longest lived isotope. Astatine: Atomic mass number given for longest lived isotope. Radon: Atomic mass number given for longest lived isotope.
Elemental Atomic Mass
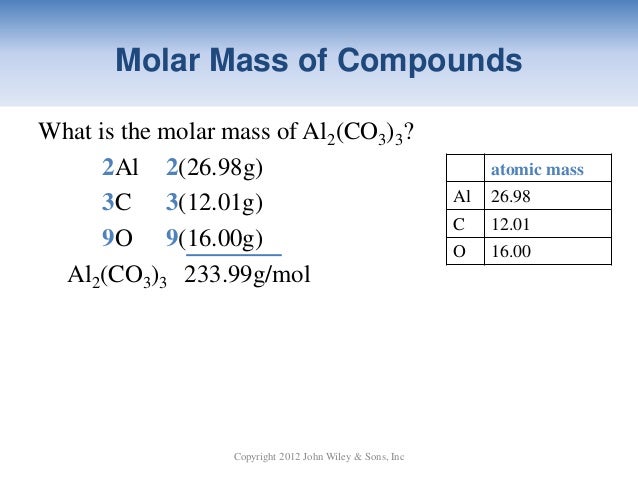
The elemenents of the periodic table sorted by atomic mass
click on any element's name for further information on chemical properties, environmental data or health effects.
This list contains the 118 elements of chemistry.
| The chemical elements of the periodic chart sorted by: | Atomic Mass | Name chemical element | Symbol | Atomic number |
| - Name alphabetically | 1.0079 | Hydrogen | H | 1 |
| - Atomic number | 4.0026 | Helium | He | 2 |
| - Symbol | 6.941 | Lithium | Li | 3 |
| - Atomic Mass | 9.0122 | Beryllium | Be | 4 |
| - Electronegativity | 10.811 | Boron | B | 5 |
| - Density | 12.0107 | Carbon | C | 6 |
| - Melting point | 14.0067 | Nitrogen | N | 7 |
| - Boiling point | 15.9994 | Oxygen | O | 8 |
| - Vanderwaals radius | 18.9984 | Fluorine | F | 9 |
| - Year of discovery | 20.1797 | Neon | Ne | 10 |
| - Inventor surname | 22.9897 | Sodium | Na | 11 |
| - Elements in earthcrust | 24.305 | Magnesium | Mg | 12 |
| - Elements in human body | 26.9815 | Aluminum | Al | 13 |
| - Covalenz radius | 28.0855 | Silicon | Si | 14 |
| - Ionization energy | 30.9738 | Phosphorus | P | 15 |
For chemistry students and teachers: The tabular chart on the right is arranged by Atomic mass (weight). The lightest chemical element is Hydrogen and the heaviest is Hassium. The unity for atomic mass is gram per mol. Please note that the elements do not show their natural relation towards each other as in the Periodic system. There you can find the metals, semi-conductor(s), non-metal(s), inert noble gas(ses), Halogens, Lanthanoides, Actinoids (rare earth elements) and transition metals. | 32.065 | Sulfur | S | 16 |
| 35.453 | Chlorine | Cl | 17 | |
| 39.0983 | Potassium | K | 19 | |
| 39.948 | Argon | Ar | 18 | |
| 40.078 | Calcium | Ca | 20 | |
| 44.9559 | Scandium | Sc | 21 | |
| 47.867 | Titanium | Ti | 22 | |
| 50.9415 | Vanadium | V | 23 | |
| 51.9961 | Chromium | Cr | 24 | |
| 54.938 | Manganese | Mn | 25 | |
| 55.845 | Iron | Fe | 26 | |
| 58.6934 | Nickel | Ni | 28 | |
| 58.9332 | Cobalt | Co | 27 | |
| 63.546 | Copper | Cu | 29 | |
| 65.39 | Zinc | Zn | 30 | |
| 69.723 | Gallium | Ga | 31 | |
| 72.64 | Germanium | Ge | 32 | |
| 74.9216 | Arsenic | As | 33 | |
| 78.96 | Selenium | Se | 34 | |
| 79.904 | Bromine | Br | 35 | |
| 83.8 | Krypton | Kr | 36 | |
| 85.4678 | Rubidium | Rb | 37 | |
| 87.62 | Strontium | Sr | 38 | |
| 88.9059 | Yttrium | Y | 39 | |
| 91.224 | Zirconium | Zr | 40 | |
| 92.9064 | Niobium | Nb | 41 | |
| 95.94 | Molybdenum | Mo | 42 | |
| 98 | Technetium | Tc | 43 | |
| 101.07 | Ruthenium | Ru | 44 | |
| 102.9055 | Rhodium | Rh | 45 | |
| 106.42 | Palladium | Pd | 46 | |
| 107.8682 | Silver | Ag | 47 | |
| 112.411 | Cadmium | Cd | 48 | |
| 114.818 | Indium | In | 49 | |
| 118.71 | Tin | Sn | 50 | |
| 121.76 | Antimony | Sb | 51 | |
| 126.9045 | Iodine | I | 53 | |
| 127.6 | Tellurium | Te | 52 | |
| 131.293 | Xenon | Xe | 54 | |
| 132.9055 | Cesium | Cs | 55 | |
| 137.327 | Barium | Ba | 56 | |
| 138.9055 | Lanthanum | La | 57 | |
| 140.116 | Cerium | Ce | 58 | |
| 140.9077 | Praseodymium | Pr | 59 | |
| 144.24 | Neodymium | Nd | 60 | |
| 145 | Promethium | Pm | 61 | |
| 150.36 | Samarium | Sm | 62 | |
| 151.964 | Europium | Eu | 63 | |
| 157.25 | Gadolinium | Gd | 64 | |
| 158.9253 | Terbium | Tb | 65 | |
| 162.5 | Dysprosium | Dy | 66 | |
| 164.9303 | Holmium | Ho | 67 | |
| 167.259 | Erbium | Er | 68 | |
| 168.9342 | Thulium | Tm | 69 | |
| 173.04 | Ytterbium | Yb | 70 | |
| 174.967 | Lutetium | Lu | 71 | |
| 178.49 | Hafnium | Hf | 72 | |
| 180.9479 | Tantalum | Ta | 73 | |
| 183.84 | Tungsten | W | 74 | |
| 186.207 | Rhenium | Re | 75 | |
| 190.23 | Osmium | Os | 76 | |
| 192.217 | Iridium | Ir | 77 | |
| 195.078 | Platinum | Pt | 78 | |
| 196.9665 | Gold | Au | 79 | |
| 200.59 | Mercury | Hg | 80 | |
| 204.3833 | Thallium | Tl | 81 | |
| 207.2 | Lead | Pb | 82 | |
| 208.9804 | Bismuth | Bi | 83 | |
| 209 | Polonium | Po | 84 | |
| 210 | Astatine | At | 85 | |
| 222 | Radon | Rn | 86 | |
| 223 | Francium | Fr | 87 | |
| 226 | Radium | Ra | 88 | |
| 227 | Actinium | Ac | 89 | |
| 231.0359 | Protactinium | Pa | 91 | |
| 232.0381 | Thorium | Th | 90 | |
| 237 | Neptunium | Np | 93 | |
| 238.0289 | Uranium | U | 92 | |
| 243 | Americium | Am | 95 | |
| 244 | Plutonium | Pu | 94 | |
| 247 | Curium | Cm | 96 | |
| 247 | Berkelium | Bk | 97 | |
| 251 | Californium | Cf | 98 | |
| 252 | Einsteinium | Es | 99 | |
| 257 | Fermium | Fm | 100 | |
| 258 | Mendelevium | Md | 101 | |
| 259 | Nobelium | No | 102 | |
| 261 | Rutherfordium | Rf | 104 | |
| 262 | Lawrencium | Lr | 103 | |
| 262 | Dubnium | Db | 105 | |
| 264 | Bohrium | Bh | 107 | |
| 266 | Seaborgium | Sg | 106 | |
| 268 | Meitnerium | Mt | 109 | |
| 272 | Roentgenium | Rg | 111 | |
| 277 | Hassium | Hs | 108 | |
| Darmstadtium | Ds | 110 | ||
| Ununbium | Uub | 112 | ||
| Ununtrium | Uut | 113 | ||
| Ununquadium | Uuq | 114 | ||
| Ununpentium | Uup | 115 | ||
| Ununhexium | Uuh | 116 | ||
| Ununseptium | Uus | 117 | ||
| Ununoctium | Uuo | 118 |
Click here: for a schematic overview of the periodic table of elements in chart form
Do you need to know the weight of some molecules? Try our Molecular Weight Calculator!
Lenntech BV
Rotterdamseweg 402 M
2629 HH Delft
tel: +31 152 610 900
fax: +31 152 616 289
e-mail: info@lenntech.com
Al Atomic Mass Amu
Atomic Mass and Nuclear Binding Energy for Al-26 (Aluminium)
Abstract
This document is part of the Supplement containing the complete sets of data of Subvolume A `Nuclei with Z = 1 - 54' of Volume 22 `Nuclear Binding Energies and Atomic Masses' of Landolt-Börnstein - Group I `Elementary Particles, Nuclei and Atoms'. It provides atomic mass, mass excess, nuclear binding energy, nucleon separation energies, Q-values, and nucleon residual interaction parameters for atomic nuclei of the isotope Al-26 (Aluminium, atomic number Z = 13, mass number A = 26).



- atomic mass;
- mass excess;
- nuclear binding energy;
- nucleon separation energy;
- Q-value;
- nucleon residual interaction parameter
